Tin is characterized by acceptable conductivity, excellent solderability, relatively low cost, a method of preventing contamination from the base metal, and softness that makes it easy to break up films with minute sliding action and relatively light loads. The different kinds of tin coatings have different rates of degradation, some have limited tin thickness, some are less porous, some diffuse faster, and some embody diffusion barriers. Some of these characteristics and all of these differences affect contact resistance.
Contact resistance of tin coatings generally increase with exposure to elevated temperatures (see Figure 5).
After 250 hours the tin on all of the samples had formed intermetallic compounds. Yet there were distinct differences; oxides, films, and surface contaminants from the tin and/or base metal are likely causes. An effective underplate (such as nickel) with a "thick" tin plate would provide better performance.
A major reason for contact failure with tin surfaces is fretting corrosion (due to small but repeated micro-motion between contacting surfaces). Higher contact force is effective in minimizing this motion (and thus the fretting corrosion), but cannot be used unless the subsequent increase in insertion/withdrawal force is acceptable.
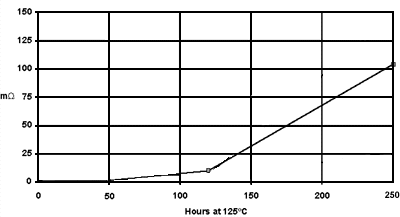 Figure 5. Contact Resistance of Tin Coated Cartridge Brass.
Figure 5. Contact Resistance of Tin Coated Cartridge Brass.